Pharmaceutical facilities need to follow and apply GxP standard and regulations. In accordance with GAMP,
FDA 21 CFR Part 11 and EU GMP Annex 11, Equipment and System Validation is obligatory.
- Validation and Qualification in Pharmaceutical Industry
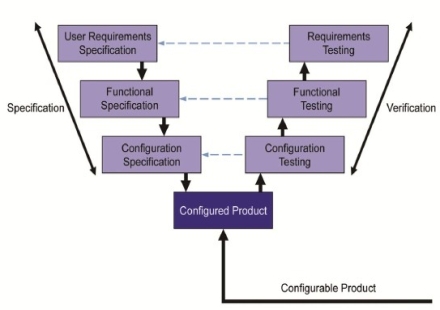
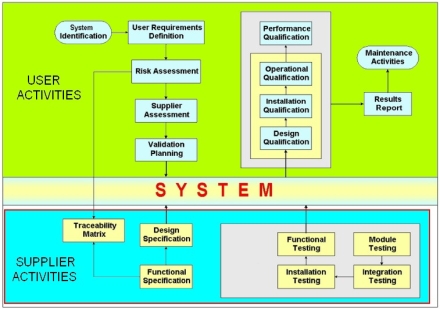
Equipment and System validation process is the documented evidence that the new or changed System
accomplishes the integrity of quality product data. Preparation and development of Qualification documentation
begins prior to Project implementation and last until the end of the Project. IT Automation experts can provide
Validation and Qualification documentation, User Requirements Specification - URS, Quality Plan - QP, Risk Analysis - RA,
Design Qualification - DQ(Functional Design Specification - FDS, Hardware Design Specification - HDS), Functional Specification - FS,
Configuration Specification - CS), Installation Qualification - IQ, Operational Specification - OQ, Performance Qualification - PQ.
|